Pharmacovigilance
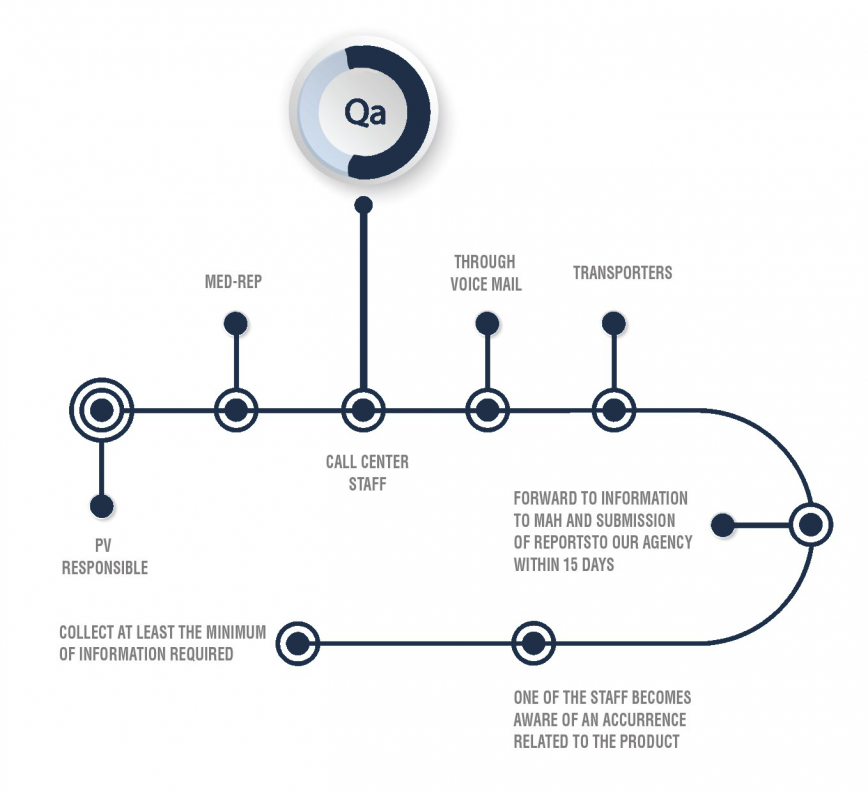
We manage product safety via the collection, detection, assessment, monitoring, reporting and prevention
Available 24/7/365
If you are encounterring adverse reactions to your treatment please contact us.
+355 (0) 42 48 64 67
[email protected]

Product Safety
We manage product safety via the collection, detection, assessment, monitoring, reporting and prevention of medicine side effects (ADR) cases as well as medicine related adverse events.
Responsibility
Our pharmacovigilance department ensures compliance with applicable regulations and/or standard operating procedures. We also offer our partners specialists in drug safety management, clinical trials and medical supervision.
Our pharmacovigilance service team among other tasks accurately archives pharmacovigilance documents; reviews safety case data and other pharmacovigilance documents for completeness and accuracy; monitors periodic safety reports through quality review of safety data and project management; assists with tracking, submitting and distribution of periodic reports; assists liaisons with the partners and cross-functional team members to ensure compliance; and supports various ad-hoc deliverables and pharmacovigilance projects.